Press Release
RepliCel Reports 2013 Annual Financial Results

VANCOUVER, BC – March 18, 2014 – RepliCel Life Sciences Inc. (OTCQB: REPCF) (TSXV: RP) a clinical stage regenerative medicine company focused on the development of autologous cell therapies, today reported financial results for the year ended December 31, 2013.
“2013 was a significant year of technology growth for the company,” said David Hall, CEO of RepliCel. “We expanded our pipeline to include a fibroblast platform based on our core competency around hair follicle mesenchymal cells. This includes a phase 2 trial for chronic tendinosis and a phase 1 trial for aging and sun damaged skin. In addition to the development of these autologous cell therapies, RepliCel has also developed a second generation dermal injector device to support its RCH-01 and RCS-01 products.”
Gemma Fetterley, VP Finance commented, “During 2013 we were very pleased to complete our company’s first non-dilutive geographic commercial license on our RCH-01 hair loss technology with Shiseido Company, Limited in the amount of $34 million including a $4.1 million upfront payment. The Company plans to seek out new geographic licensing opportunities on RCH-01 and our other technologies as we advance into and through the clinical regulatory process. RepliCel exited 2013 with $2.0 million in cash to support on-going research and development.”
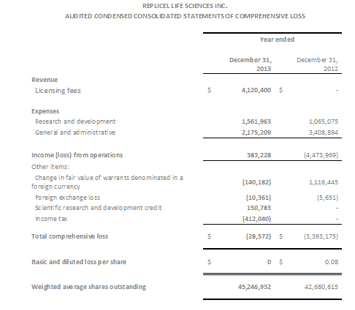
2013 Year End Financial Results (CDN $)
Net loss for the year ended December 31, 2013 was $28,572 or $nil per share compared to a net loss of $3,363,175 or $0.08 per share for the comparable year ended 2012.
Revenue for the year ended December 31, 2013, was $4,120,400, attributed to completion of a Collaboration and Technology Development Transfer Agreement with Shiseido Company, Limited. There was no revenue from operations for the year ended December 31, 2012.
Research and Development expenses totaled $1,561,963 for the year ended December 31, 2013 compared to $1,065,075 for the year ended December 31, 2012. The increase was the result of advancing the pre-clinical work for RCT-01, development of the RCI-02 injector device prototype, improvements in the cell replication process for RCH-01 in preparation for our submission to regulatory authorities and incremental expenditure on intellectual property.
General and administrative expenses totalled $2,175,209 for the year ended December 31, 2013 compared to $3,408,894 for the year ended December 31, 2012. The decrease is primarily attributable to a decline in marketing and investor relations activities, and a reduction in stock based compensation which resulted from the forfeiture of options held by certain employees and consultants during the year.
RepliCel held $2.0 million of cash and cash equivalents as at December 31, 2013. This compares to cash and cash equivalents of $0.4 million at December 31, 2012.
Notice Regarding Forward Looking Statements
This press release contains projections and forward-looking statements, as that term is defined under applicable securities laws. Statements in this press release, which are not purely historical, are forward-looking statements that relate to the approval and commercialization of the Company’s hair restoration process, the Company’s development of its Achilles tendon technology and the expected commencement of the Company’s Phase 2 clinical trial for its tendon technology. These statements are only predictions and involve known and unknown risks which may cause actual results and the Company’s plans and objectives to differ materially from those expressed in the forward-looking information, including: negative results from the Company’s clinical trials; the effects of government regulation on the Company’s business; risks associated with the Company’s ability to obtain and protect rights to its intellectual property; risks and uncertainties associated with the Company’s ability to raise additional capital; and other factors beyond the Company’s control. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, it cannot guarantee future results, levels of activity or performance. Further, any forward-looking statement speaks only as of the date on which such statement is made, and, except as required by applicable law, the Company undertakes no obligation to update any forward-looking statement to reflect events or circumstances after the date on which such statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for management to predict all of such factors and to assess in advance the impact of such factors on the Company’s business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statement. Readers should consult all of the information set forth herein and should also refer to the risk factor disclosure outlined in the Company’s annual report on Form 20-F for the fiscal year ended December 31, 2013 and other periodic reports filed from time-to-time with the Securities and Exchange Commission on Edgar at www.sec.gov and with the British Columbia Securities Commission on Sedar at www.sedar.com.
CORPORATE CONTACTS:
David M. Hall, Chief Executive Officer
Tammey George, Director of Communications
RepliCel Life Sciences
604-248-8696
tg@replicel.com
INVESTOR CONTACT:
Westwicke Partners, LLC
Robert H. Uhl
Managing Director
(858) 356-5932
robert.uhl@westwicke.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
by Topic
DISCLAIMER:
The information in these press releases is historical in nature, has not been updated, and is current only to the date indicated in the particular press release. This information may no longer be accurate and therefore you should not rely on the information contained in these press releases. To the extent permitted by law, RepliCel Life Sciences Inc. and its employees, agents and consultants exclude all liability for any loss or damage arising from the use of, or reliance on, any such information, whether or not caused by any negligent act or omission.
THIRD PARTY CONTENT
Please note that any opinion, estimates or forecasts made by the authors of these statements are theirs alone and do not represent opinions, forecasts or predictions of RepliCel Life Sciences Inc. or its management. RepliCel Life Sciences Inc. does not, by its reference or distribution of these links imply its endorsement of, or concurrence with, such information, conclusions or recommendations.