As Seen In
Impact Magazine: Aching Achilles?

Regenerative Medicine Promises New Cure for Chronic Tendon Injuries
By Dr. Jack Taunton
A Regerative Cell therapy is now being tested on people suffering from chronic Achilles tendinosis at the Sport Medicine Clinic at the University of British Columbia. ________________________________________________________________________
 Clinical researchers are testing a treatment developed by RepliCel Life Sciences called RCT-01 which is manufactured personally for each patient using specialized cells isolated from their own hair follicles. Several weeks after the collection of a single-suture biopsy taken from the back of the patient’s scalp, the product, a suspension of their own cells, is injected, under ultrasound guidance, into the injured tendon to promote its repair, Capturerestore function and eliminate pain.
Clinical researchers are testing a treatment developed by RepliCel Life Sciences called RCT-01 which is manufactured personally for each patient using specialized cells isolated from their own hair follicles. Several weeks after the collection of a single-suture biopsy taken from the back of the patient’s scalp, the product, a suspension of their own cells, is injected, under ultrasound guidance, into the injured tendon to promote its repair, Capturerestore function and eliminate pain.
It is exciting that following nearly 20 years of tendinopathy research, the medical community, at last, may have an answer for patients with chronic tendinopathy which current treatments simply cannot address.
Achilles tendinosis is a painful overuse injury that affects the lower leg. Often 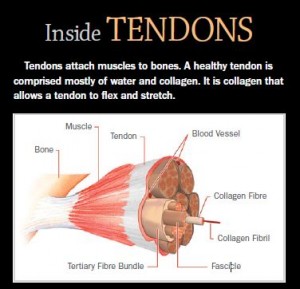 caused by a cycle of injury, improper healing and re-injury, chronic tendinosis is an accumulation of micro-tears which leads to structural degeneration of the tendon associated with weakness, loss of function, chronic pain and susceptibility to re-tearing and further injury.
caused by a cycle of injury, improper healing and re-injury, chronic tendinosis is an accumulation of micro-tears which leads to structural degeneration of the tendon associated with weakness, loss of function, chronic pain and susceptibility to re-tearing and further injury.
Physiotherapy and dextrose injections are the most common treatments, but often don’t heal all the micro-tears. Up to 30 per cent of patients don’t satisfactorily improve despite following the prescribed protocols.
This is where the injection under ultrasound guidance comes into play. RCT-01 is a cell therapy product made from fibroblast cells that express high levels of Type 1 collagen – the key and missing ingredient needed to permanently heal the micro-tears and fully repair the tendon. In an injured tendon, these fibroblasts (or tenocytes as they are called when resident in a tendon) are simply exhausted and incapable of the Type 1 collagen production needed, or lay down the weak Type 3 collagen highly susceptible to re-tearing. RCT-01 is an ultrasound guided injection of fresh fibroblasts highly expressive of the Type 1 collagen needed to permanently repair the tendon and return the patient to full function.
RepliCel plans to expand clinical testing of its product to other areas of tendinosis (e.g., knee, elbow, etc.) as well as investigating both the treatment’s long-term durability and its potential to treat the more acute and inflammatory tendinitis, which often precedes the chronic condition.
To learn more, visit www.tendonstudy.com
Find the full article in Impact Magazine on Pg. 78.
by Topic
DISCLAIMER:
The information in these press releases is historical in nature, has not been updated, and is current only to the date indicated in the particular press release. This information may no longer be accurate and therefore you should not rely on the information contained in these press releases. To the extent permitted by law, RepliCel Life Sciences Inc. and its employees, agents and consultants exclude all liability for any loss or damage arising from the use of, or reliance on, any such information, whether or not caused by any negligent act or omission.
THIRD PARTY CONTENT
Please note that any opinion, estimates or forecasts made by the authors of these statements are theirs alone and do not represent opinions, forecasts or predictions of RepliCel Life Sciences Inc. or its management. RepliCel Life Sciences Inc. does not, by its reference or distribution of these links imply its endorsement of, or concurrence with, such information, conclusions or recommendations.