As Seen In
Cell Therapy is “Hair Raising” in a Good Way

RepliCel Targets Low-Risk, Near-Term Opportunities in Regenerative Medicine
By: Gail Dutton
The regenerative medicine company RepliCel Life Sciences is developing potential cures for chronic tendinosis, damaged or aging skin, and pattern baldness by reseeding affected areas with specific cell populations isolated from patients’ own healthy hair follicles.
RepliCel is picking the low-hanging fruit of regenerative medicine—low technological risk, underserved markets, clear clinical indications. Furthermore, commercial success is not dependent on successful reimbursement negotiations.
“On the technical level, we’re not asking these cells to do anything other than what they naturally do, or be anything more than they are,” CEO David Hall says. “These are adult, somatic cells derived from the patient which we simply isolate and grow. We’re not differentiating, genetically modifying, or manipulating these cells in any way.
 At present, there is no therapy to treat the underlying causes of tendinosis, which include a deficit of collagen-producing tenocytes (fibroblasts). RepliCel’s approach to the treatment of tendinosis uses injections of cultured autologous, collagen-producing, nonbulbar dermal sheath cells into the injury site to stimulate tendon regeneration
At present, there is no therapy to treat the underlying causes of tendinosis, which include a deficit of collagen-producing tenocytes (fibroblasts). RepliCel’s approach to the treatment of tendinosis uses injections of cultured autologous, collagen-producing, nonbulbar dermal sheath cells into the injury site to stimulate tendon regeneration
From the scientific and manufacturing perspective, RepliCel is using the hair follicle as the cell source because the cells are simple to collect, grow well in culture, and are both relatively naive and highly functional. On a clinical level, the company is simply addressing a deficit of active cells in the patient by local delivery of cells shown to function in ways needed to solve a human condition such as tendinosis or pattern baldness.
“The cells are injected in ways and places that largely eliminate any concerns around in vivo cell migration,” explains Hall. “[This approach ensures] enough cells stay in situ and viable to affect a sustained effect.”
Indications
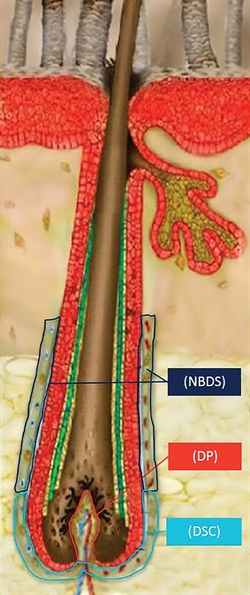
For tendinosis—a disrupted healing cycle of the tendon—nonbulbar dermal sheath (NBDS) fibroblast cells are isolated from a biopsy of hair follicles taken from the back of the scalp. After these cells are replicated, creating populations of millions of cells, they are injected into the wound site to jump-start the disrupted wound repair.
In early-stage trials, clinical advisory board member David Connell, M.D., used a similar approach with tendinosis patients who had been failed by other therapies. The NBDS approach returned these patients to painless, near-normal function.
In the next 18 months, Hall says he expects to conclude a Phase I/II study at the University of British Columbia involving 28 participants. As yet, there is no approved therapy that treats the underlying cause of this chronic condition.
This same NBDS platform technology also may be used to repair damaged and aging skin. RepliCel filed a clinical trial application for Germany in February.
Phase II trials to treat baldness—specifically, androgenetic alopecia—will begin this year. For this therapy, dermal sheath cup (DSC) cells are isolated from the base of the hair follicle, replicated into the millions, and injected to the area of thinning hair.
“DSC cells are responsible for maintaining the number of dermal papillae cells, which directly corresponds to the hair thickness,” Hall explains. “We are simply delivering a volume of androgen-insensitive DSC cells into an area where androgen-sensitive DSC cells have disappeared … to restore the normal hair follicle cycle.”
RepliCel’s autologous cell therapy utilizes dermal sheath cup (DSC) cells to treat androgenetic alopecia. DSC cells are responsible for the regulation of the volume of dermal papillae (DP) cells, which are responsible for the thickness and growth of a hair fiber. Another RepliCel technology uses nonbulbar dermal sheath (NBDS) fibroblast cells to treat tendinosis.
 In animal studies, this approach grew hair on the feet of mice (which have no hair follicles there). When these cells were injected into their ears, the healthy cells migrated into resident hair follicles, making that hair thicker.
In animal studies, this approach grew hair on the feet of mice (which have no hair follicles there). When these cells were injected into their ears, the healthy cells migrated into resident hair follicles, making that hair thicker.
In humans, this approach could address existing hair follicle damage. “This is our longer-term program,” Hall stresses. RepliCel will begin a clinical trial in Germany this year involving 160 patients. Hall says results from RepliCel’s Phase I and Phase II clinical trials for chronic tendinosis and UV-damaged/aged skin are expected in 2016.
Manufacturing and Delivery
RepliCel currently uses industry-standard fetal calf serum in its manufacturing process, but is validating a serum-free process. “This is a distinguishing commercial step,” Hall asserts. “[It] adds value to our therapeutics program.” Hall notes that RepliCel anticipates licensing the process.
RepliCel is also investigating the potential of using its cells in an allogeneic setting. Hall says that a successful licensing effort could enhance both the business model and the value of company assets.
RepliCel also is developing a dermal injector to ensure controlled, consistent delivery of the cells to the skin and scalp. RepliCel expects that this device will control cell volume and delivery depth while minimizing shear force to the cells.
“We have also incorporated a Pelletier element into the tip, which reduces or removes the need for pre-injection anesthetics,” Hall informs. This second-generation injector is available for licensure. RepliCel plans to file for the CE mark in the latter half of 2015.
Partnerships
RepliCel already has a partnership with Shiseido in Japan to co-develop and commercialize RepliCel’s RCH-01 technology as a therapy for thinning hair in China, South Korea, and the ASEAN nations. RepliCel is actively pursuing similar geographically focused licensing and co-development partnerships for its other products.
It also is commercially active in Japan, where recent changes to Japanese regulations for regenerative medicine “have dramatically changed the pathway toward revenue generation,” Hall remarks. Those changes allow products that have demonstrated safety and potential efficacy in early-stage trials to gain conditional marketing approval. That lets them be sold and reimbursed up to seven years before being subjected to a final marketing approval review.
“In the current Japanese environment, we can generate data while simultaneously generating revenue and continuing to develop the same technology in the West,” Hall confides. He suggests that as a result of these regulatory changes, Japan will become important as a test bed for regenerative medicine.
RepliCel’s business model seems well-suited to Japan’s new regulatory stance for regenerative medicine. “We’re built to discover, validate, and license,” Hall states. “Commercialization is done with partners.”
by Topic
DISCLAIMER:
The information in these press releases is historical in nature, has not been updated, and is current only to the date indicated in the particular press release. This information may no longer be accurate and therefore you should not rely on the information contained in these press releases. To the extent permitted by law, RepliCel Life Sciences Inc. and its employees, agents and consultants exclude all liability for any loss or damage arising from the use of, or reliance on, any such information, whether or not caused by any negligent act or omission.
THIRD PARTY CONTENT
Please note that any opinion, estimates or forecasts made by the authors of these statements are theirs alone and do not represent opinions, forecasts or predictions of RepliCel Life Sciences Inc. or its management. RepliCel Life Sciences Inc. does not, by its reference or distribution of these links imply its endorsement of, or concurrence with, such information, conclusions or recommendations.